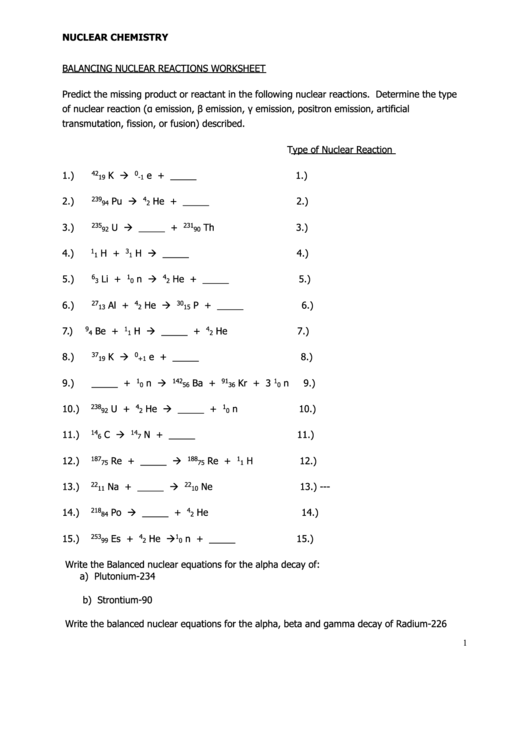
Balancing Chemical Equations Coloring Answer Key Pdf - 2 ag 2 o → 4 ag + 1 o 2 4. The equation identifies the reactants (starting materials) and products. 1 s 8 + 12 o 2 → 8. 1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl + 3 o2 3) 2 nacl + 1 f2 æ. A chemical equation describes what happens in a chemical reaction. Shade in the answer box with the color given & then color the. 4 p + 5 o 2 → 2 p 2 o 5 2. Determine whether the following equations are balanced or unbalanced. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. 2 na + 2 h 2 o → 2 naoh + 1 h 2 3.
The equation identifies the reactants (starting materials) and products. Determine whether the following equations are balanced or unbalanced. Write the word equations below as chemical equations and balance: 2 ag 2 o → 4 ag + 1 o 2 4. Shade in the answer box with the color given & then color the. A chemical equation describes what happens in a chemical reaction. 1 s 8 + 12 o 2 → 8. 1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl + 3 o2 3) 2 nacl + 1 f2 æ. 4 p + 5 o 2 → 2 p 2 o 5 2. 2 na + 2 h 2 o → 2 naoh + 1 h 2 3.
2 ag 2 o → 4 ag + 1 o 2 4. Write the word equations below as chemical equations and balance: 4 p + 5 o 2 → 2 p 2 o 5 2. Shade in the answer box with the color given & then color the. Determine whether the following equations are balanced or unbalanced. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. A chemical equation describes what happens in a chemical reaction. 2 na + 2 h 2 o → 2 naoh + 1 h 2 3. The equation identifies the reactants (starting materials) and products. 1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl + 3 o2 3) 2 nacl + 1 f2 æ.
Balancing Chemical Equations Worksheet 1 Answer Key Equations Worksheets
1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl + 3 o2 3) 2 nacl + 1 f2 æ. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. A chemical equation describes what happens in a chemical reaction. 2 na + 2 h 2 o → 2 naoh + 1.
Answer Key Chemistry Answer Key Balancing Equations Workshee
1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl + 3 o2 3) 2 nacl + 1 f2 æ. 1 s 8 + 12 o 2 → 8. 2 na + 2 h 2 o → 2 naoh + 1 h 2 3. 4 p + 5 o 2 → 2 p 2 o.
Free Balancing Chemical Equations Worksheet [PDF] With Answers
4 p + 5 o 2 → 2 p 2 o 5 2. 1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl + 3 o2 3) 2 nacl + 1 f2 æ. 2 na + 2 h 2 o → 2 naoh + 1 h 2 3. Determine whether the following equations are balanced.
Balancing Equations 44 Download Free PDF Chemistry
1 s 8 + 12 o 2 → 8. Write the word equations below as chemical equations and balance: 4 p + 5 o 2 → 2 p 2 o 5 2. A chemical equation describes what happens in a chemical reaction. Determine whether the following equations are balanced or unbalanced.
Balancing Chemical Equations Phet Key
2 na + 2 h 2 o → 2 naoh + 1 h 2 3. 2 ag 2 o → 4 ag + 1 o 2 4. Shade in the answer box with the color given & then color the. Determine whether the following equations are balanced or unbalanced. Write the word equations below as chemical equations and balance:
Free Balancing Chemical Equations Worksheet [PDF] With Answers
4 p + 5 o 2 → 2 p 2 o 5 2. 2 na + 2 h 2 o → 2 naoh + 1 h 2 3. Shade in the answer box with the color given & then color the. Write the word equations below as chemical equations and balance: 1) 1 n2 + 3 h2 æ 2 nh3.
Free Balancing Chemical Equations Worksheet [PDF] With Answers
A chemical equation describes what happens in a chemical reaction. 2 na + 2 h 2 o → 2 naoh + 1 h 2 3. Shade in the answer box with the color given & then color the. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. 1 s 8 + 12 o 2 → 8.
49 Balancing Chemical Equations Worksheets [with Answers]
Write the word equations below as chemical equations and balance: 2 ag 2 o → 4 ag + 1 o 2 4. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. Determine whether the following equations are balanced or unbalanced. Shade in the answer box with the color given & then color the.
Free Balancing Chemical Equations Worksheet [PDF] With Answers
1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. A chemical equation describes what happens in a chemical reaction. The equation identifies the reactants (starting materials) and products. 2 ag 2 o → 4 ag + 1 o 2 4. 1) 1 n2 + 3 h2 æ 2 nh3 2) 2 kclo3 æ 2 kcl +.
1) 1 N2 + 3 H2 Æ 2 Nh3 2) 2 Kclo3 Æ 2 Kcl + 3 O2 3) 2 Nacl + 1 F2 Æ.
2 ag 2 o → 4 ag + 1 o 2 4. Shade in the answer box with the color given & then color the. 1) zinc and lead (ii) nitrate react to form zinc nitrate and lead. A chemical equation describes what happens in a chemical reaction.
The Equation Identifies The Reactants (Starting Materials) And Products.
Determine whether the following equations are balanced or unbalanced. 1 s 8 + 12 o 2 → 8. Write the word equations below as chemical equations and balance: 2 na + 2 h 2 o → 2 naoh + 1 h 2 3.


![Free Balancing Chemical Equations Worksheet [PDF] With Answers](https://www.typecalendar.com/wp-content/uploads/2023/04/balancing-chemical-equations-coloring-worksheet-answer-key.jpg?gid=47)


![Free Balancing Chemical Equations Worksheet [PDF] With Answers](https://www.typecalendar.com/wp-content/uploads/2023/04/answer-key-phet-balancing-chemical-equations-worksheet-answers.jpg?gid=47)
![Free Balancing Chemical Equations Worksheet [PDF] With Answers](https://www.typecalendar.com/wp-content/uploads/2023/04/balancing-chemical-equations-coloring-worksheet-answer-key-snowman.jpg?gid=47)
![49 Balancing Chemical Equations Worksheets [with Answers]](https://templatelab.com/wp-content/uploads/2017/01/balancing-equations-37.jpg)
![Free Balancing Chemical Equations Worksheet [PDF] With Answers](https://www.typecalendar.com/wp-content/uploads/2023/04/phet-balancing-chemical-equations-worksheet-answers-pdf.jpg?gid=47)