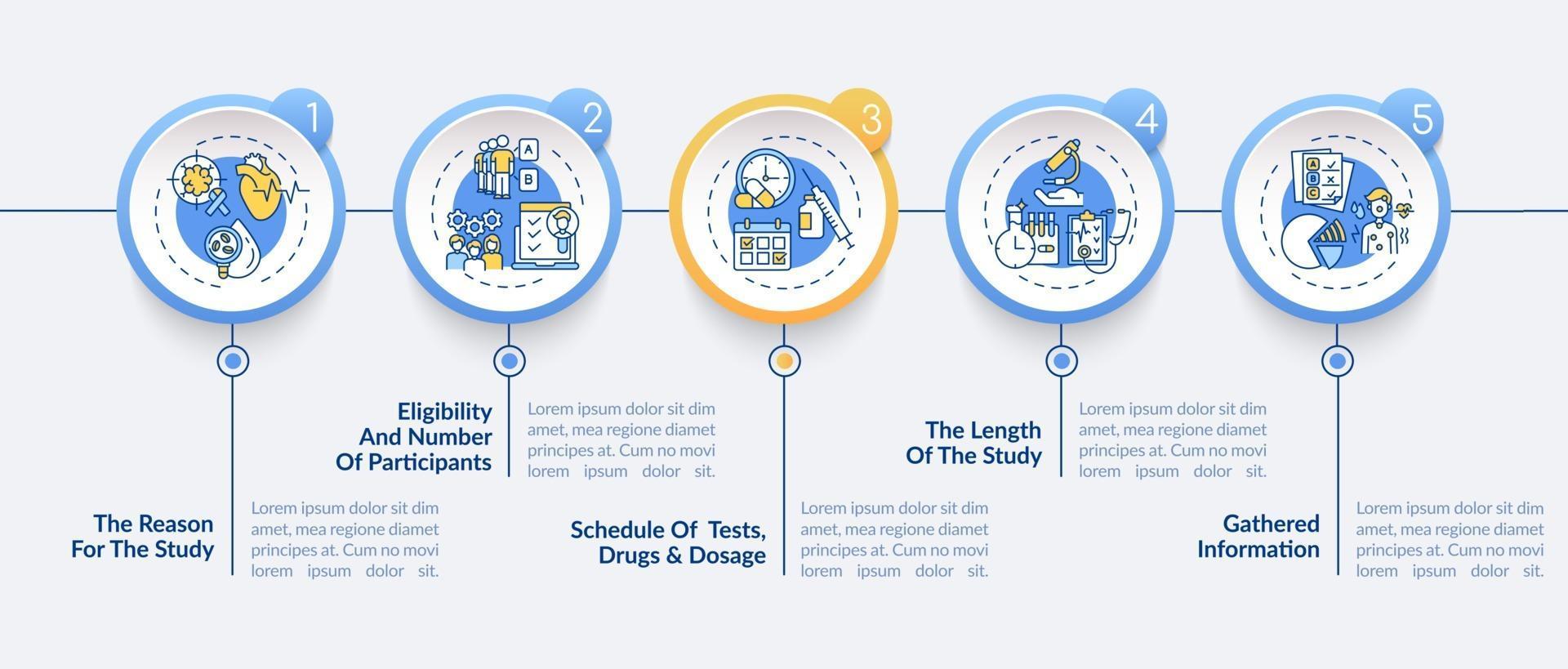
Clinical Study Protocol Template - Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. The natural history/observational protocol template, the. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Please note that this page has been updated for 2015 following a quality check. Welcome to global health trials' tools and templates library. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can. There are three templates to be used for observational research: The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and.
The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can. There are three templates to be used for observational research: Welcome to global health trials' tools and templates library. The natural history/observational protocol template, the. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. Please note that this page has been updated for 2015 following a quality check.
The natural history/observational protocol template, the. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. Welcome to global health trials' tools and templates library. Please note that this page has been updated for 2015 following a quality check. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. There are three templates to be used for observational research:
Clinical Study Protocol (CSP) Template Clinical Study Templates
There are three templates to be used for observational research: Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can. Welcome to global health trials' tools and templates library. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and..
Clinical trial protocol vector infographic template 2308775 Vector Art
Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can. Welcome to global health trials' tools and templates library. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. Nih applicants can use a template with instructional and sample.
Clinical Study Protocol Template
The natural history/observational protocol template, the. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Please note that this page has been updated for 2015 following a quality check. Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development.
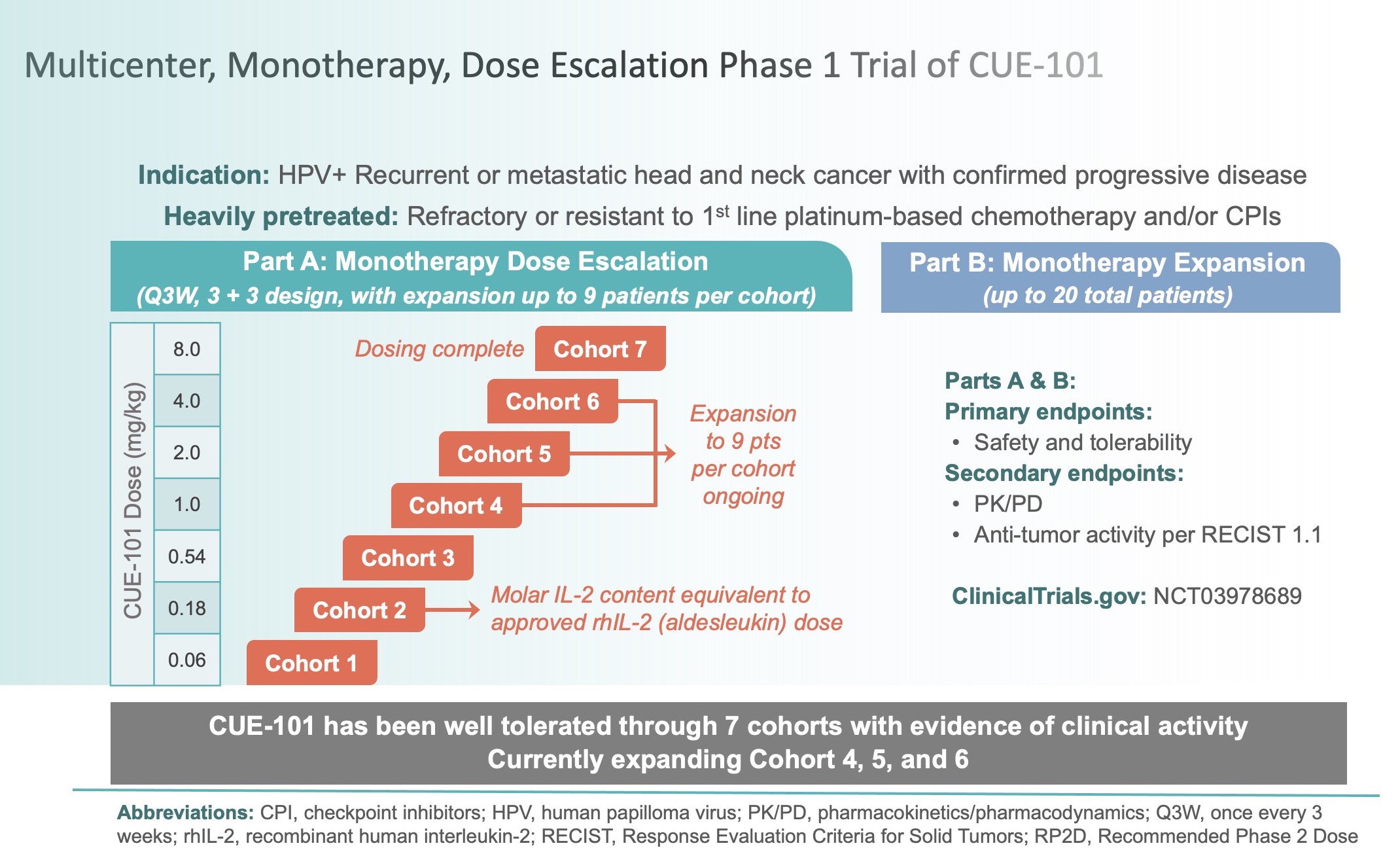
Phase 1 Clinical Trial Protocol Template
The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. There are three templates to be used for observational research: The natural history/observational protocol template, the. Please note that this.
Clinical Trial Protocol Template Word
Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Welcome to global health trials' tools and templates library. The natural history/observational protocol template, the. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. Research study protocol template (for.
Phase 1 Clinical Trial Protocol Template
Welcome to global health trials' tools and templates library. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Research study protocol template (for clinical trials) instructions this protocol template.
Phase 1 Clinical Trial Protocol Template
Welcome to global health trials' tools and templates library. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can. There are three templates to be used for observational research: Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development.
Clinical Trial Protocol Template Word
The natural history/observational protocol template, the. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. Please note that this page has been updated for 2015 following a quality check. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that.
Clinical Study Protocol Template
Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. The natural history/observational protocol template, the. There are three templates to be used for observational research: Please note that this page has been updated for 2015 following a quality check. The study designs page includes numerous basic journal articles.
Clinical Study Protocol Template
Research study protocol template (for clinical trials) instructions this protocol template is a tool to facilitate the development of a research. Welcome to global health trials' tools and templates library. Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. The study designs page includes numerous basic journal.
Please Note That This Page Has Been Updated For 2015 Following A Quality Check.
Nih applicants can use a template with instructional and sample text to help write clinical protocols for the following types of research:. There are three templates to be used for observational research: Welcome to global health trials' tools and templates library. Many recent studies have shown that highly selective biologic drugs are not effective in every patient and that variations in the genome can.
Research Study Protocol Template (For Clinical Trials) Instructions This Protocol Template Is A Tool To Facilitate The Development Of A Research.
The natural history/observational protocol template, the. The study designs page includes numerous basic journal articles linked to pubmed, introductory books on clinical epidemiology and.