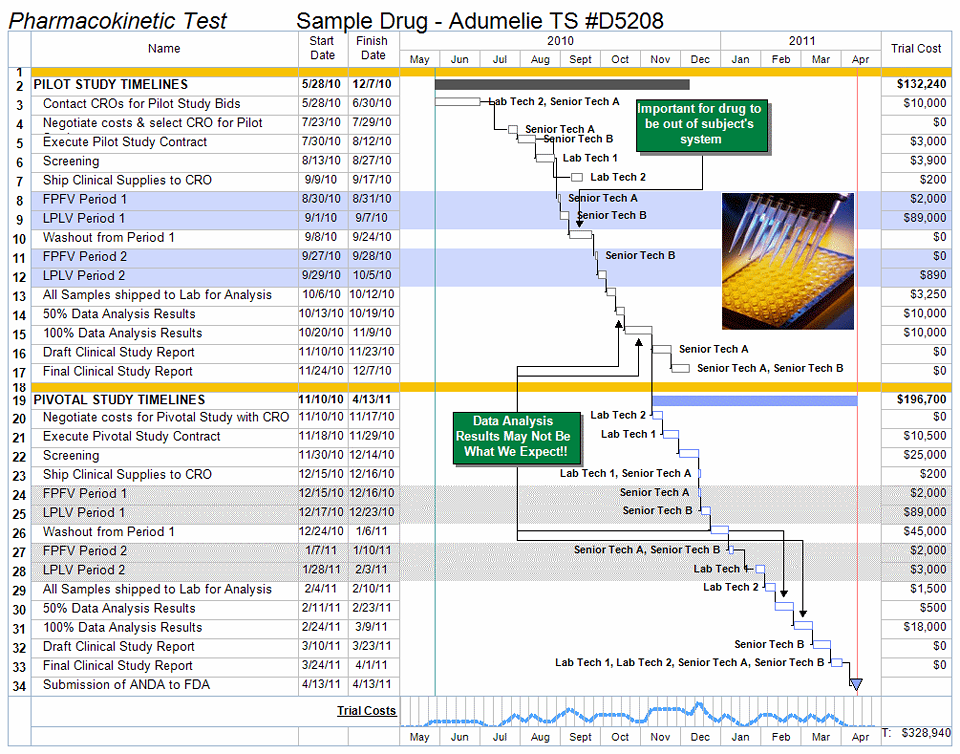
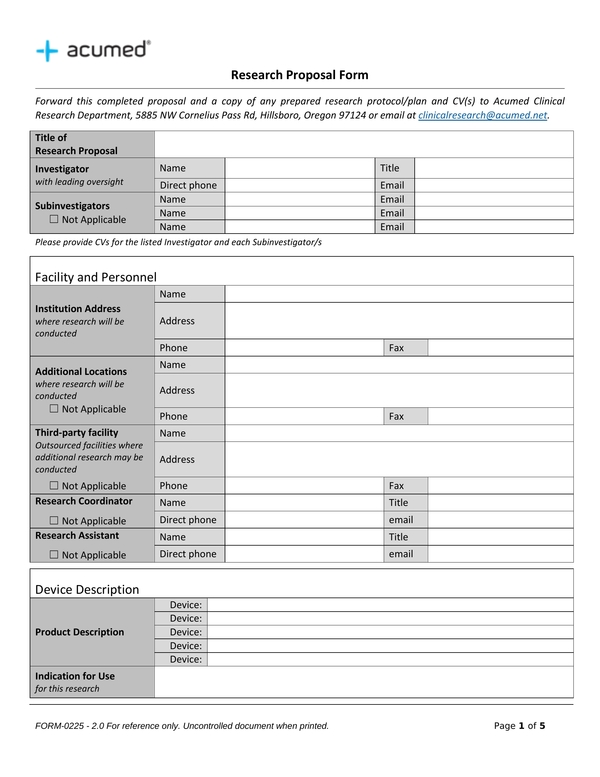
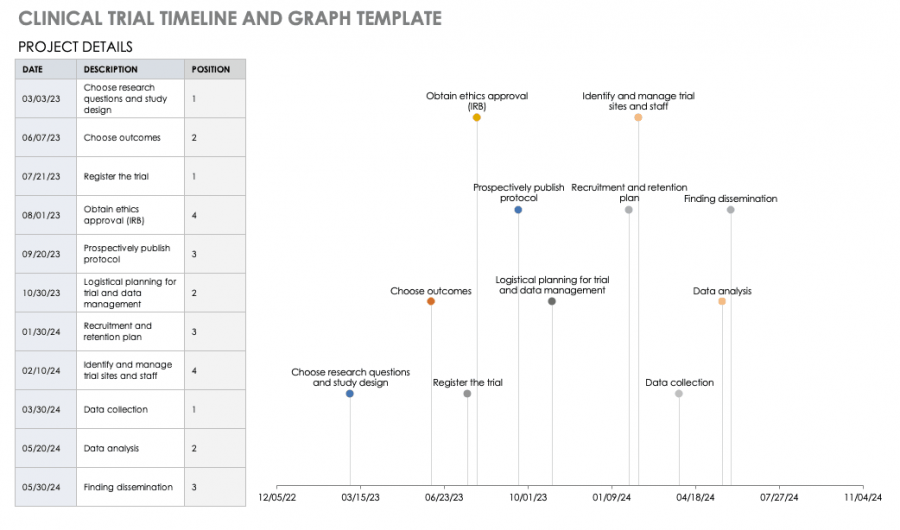
Clinical Trial Project Management Plan Template - Clinical monitoring and data management plan (cmp/dmp) checklist. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. This checklist is a tool to assist applicants and. Please note that this page has been updated for 2015 following a quality check. Investigational product accountability, delegation of authority log, adverse event reporting, and more. Welcome to global health trials' tools and templates library.
Investigational product accountability, delegation of authority log, adverse event reporting, and more. Welcome to global health trials' tools and templates library. This checklist is a tool to assist applicants and. Clinical monitoring and data management plan (cmp/dmp) checklist. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Please note that this page has been updated for 2015 following a quality check.
This checklist is a tool to assist applicants and. Welcome to global health trials' tools and templates library. Please note that this page has been updated for 2015 following a quality check. Clinical monitoring and data management plan (cmp/dmp) checklist. Investigational product accountability, delegation of authority log, adverse event reporting, and more. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an.
Clinical Research Project Plan 11+ Examples, Format, Tips, Pdf
Investigational product accountability, delegation of authority log, adverse event reporting, and more. Welcome to global health trials' tools and templates library. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Clinical monitoring and data management plan (cmp/dmp) checklist. Please note that this page has been updated for 2015.
Free Clinical Trial Project Management Plan Template Edit Online
Welcome to global health trials' tools and templates library. Clinical monitoring and data management plan (cmp/dmp) checklist. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Investigational product accountability, delegation of authority log, adverse event reporting, and more. Please note that this page has been updated for 2015.
Clinical Trial Project Management Plan Template Best Template Ideas
Welcome to global health trials' tools and templates library. Investigational product accountability, delegation of authority log, adverse event reporting, and more. Clinical monitoring and data management plan (cmp/dmp) checklist. Please note that this page has been updated for 2015 following a quality check. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial.
Clinical Research Project Plan 11+ Examples, Format, Tips, Pdf
Welcome to global health trials' tools and templates library. Investigational product accountability, delegation of authority log, adverse event reporting, and more. This checklist is a tool to assist applicants and. Clinical monitoring and data management plan (cmp/dmp) checklist. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an.
Clinical Research Project Plan 11+ Examples, Format, Tips, Pdf
Welcome to global health trials' tools and templates library. Investigational product accountability, delegation of authority log, adverse event reporting, and more. Clinical monitoring and data management plan (cmp/dmp) checklist. Please note that this page has been updated for 2015 following a quality check. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial.
Clinical Trial Project Management Plan Template
Welcome to global health trials' tools and templates library. Investigational product accountability, delegation of authority log, adverse event reporting, and more. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Please note that this page has been updated for 2015 following a quality check. This checklist is a.
FREE Project Plan Templates Edit Online & Download
This checklist is a tool to assist applicants and. Welcome to global health trials' tools and templates library. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Please note that this page has been updated for 2015 following a quality check. Clinical monitoring and data management plan (cmp/dmp).
Clinical trial project management plan template
Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Welcome to global health trials' tools and templates library. Clinical monitoring and data management plan (cmp/dmp) checklist. This checklist is a tool to assist applicants and. Investigational product accountability, delegation of authority log, adverse event reporting, and more.
Free Clinical Trial Templates Smartsheet
Please note that this page has been updated for 2015 following a quality check. Clinical monitoring and data management plan (cmp/dmp) checklist. Investigational product accountability, delegation of authority log, adverse event reporting, and more. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Welcome to global health trials'.
Clinical Trial Project Management Plan Template
Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Welcome to global health trials' tools and templates library. Clinical monitoring and data management plan (cmp/dmp) checklist. Please note that this page has been updated for 2015 following a quality check. Investigational product accountability, delegation of authority log, adverse.
Welcome To Global Health Trials' Tools And Templates Library.
Investigational product accountability, delegation of authority log, adverse event reporting, and more. This checklist is a tool to assist applicants and. Clinical trial protocol template this protocol template is designed to help research teams develop a clinical trial protocol that includes an. Please note that this page has been updated for 2015 following a quality check.