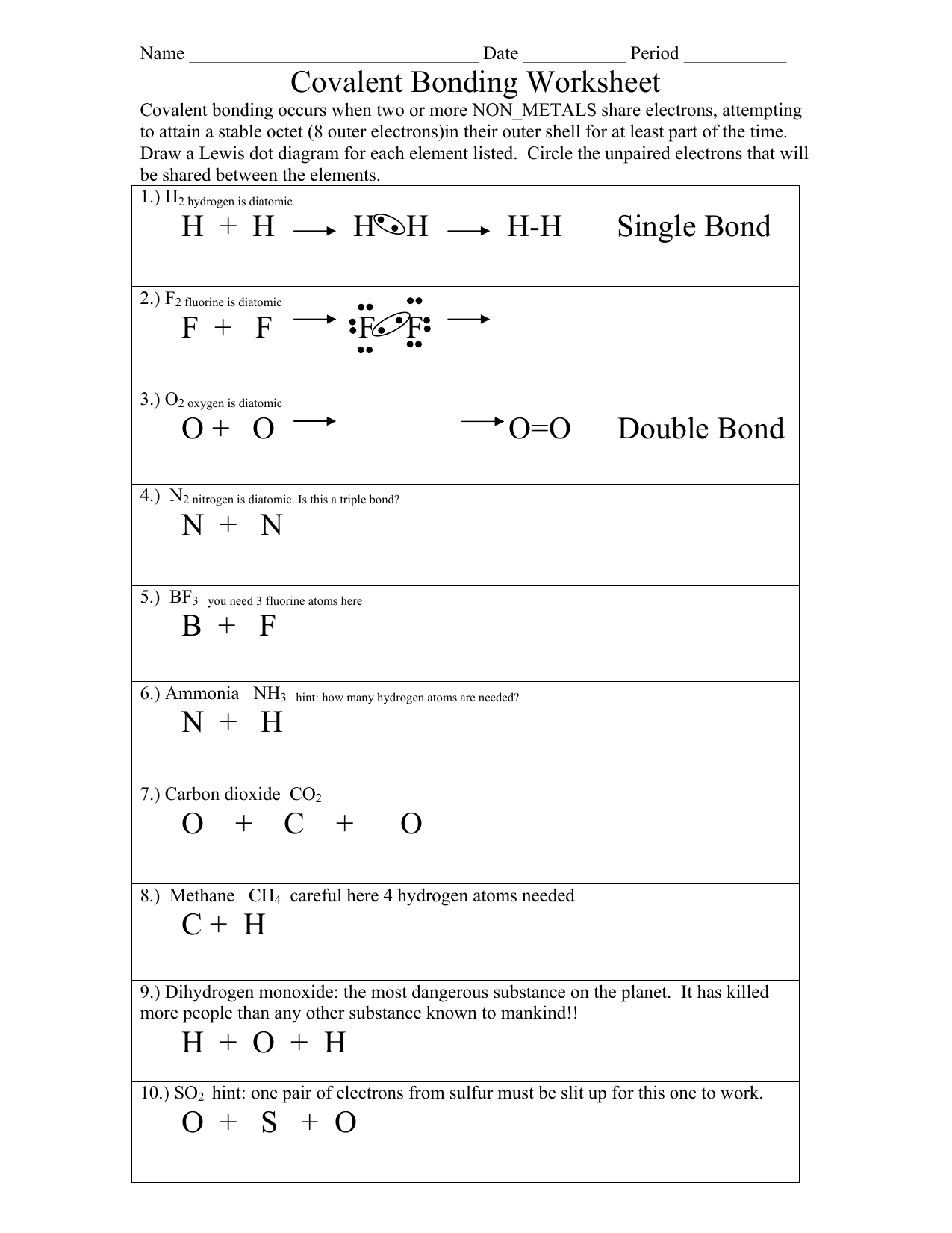
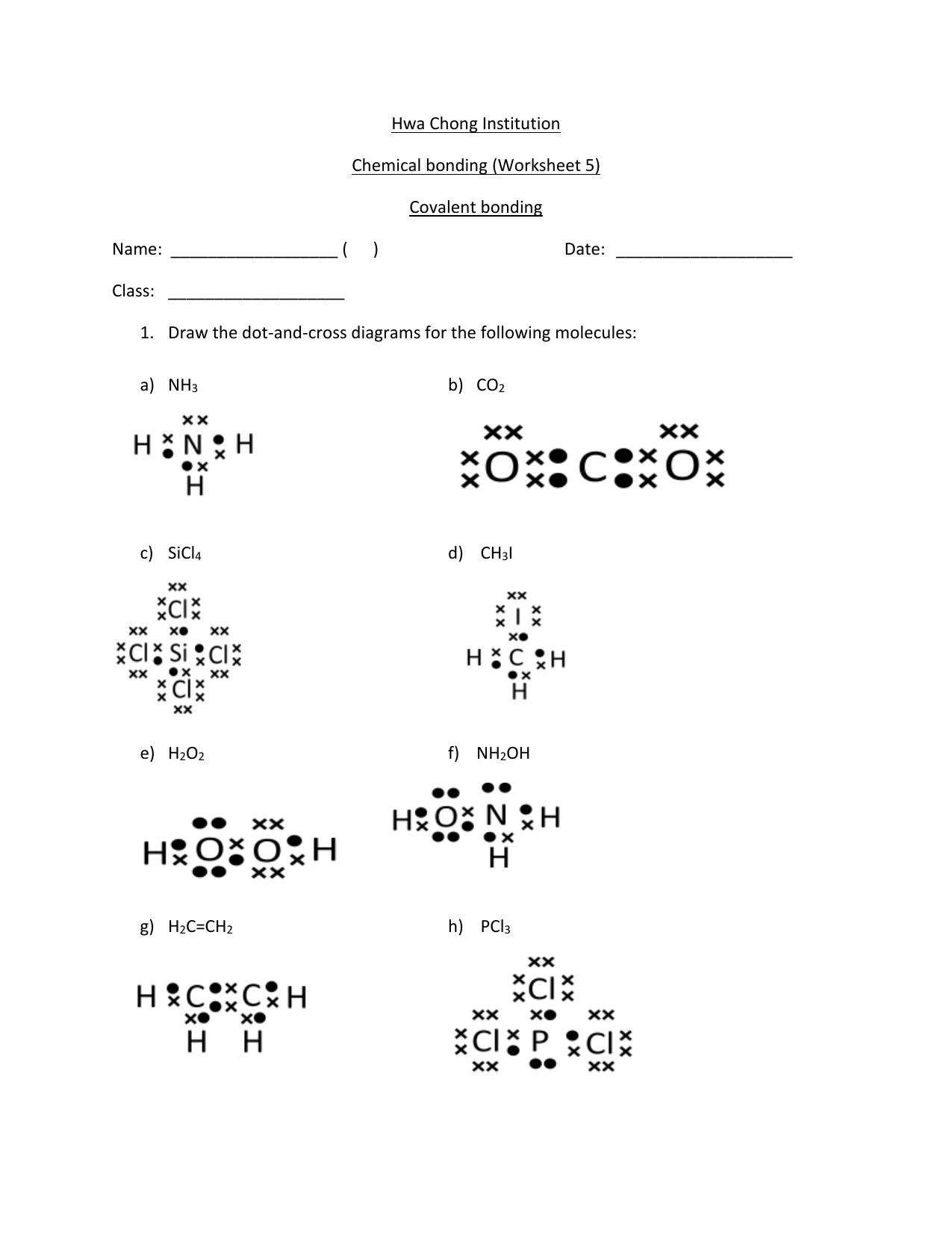
Drawing Covalent Bonds Worksheet - Why is the water molecule’s shape bent and not linear? Draw the molecular structure of water and label the partial charges of each element. Draw lewis structures for the following covalent compounds: Chemistry worksheet lewis dot structures name: Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Draw the molecular structure of water and label the partial charges of each element. Why is the water molecule’s shape bent and not linear? Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow. Draw lewis structures for the following covalent compounds: Chemistry worksheet lewis dot structures name:
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow. Why is the water molecule’s shape bent and not linear? Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Draw lewis structures for the following covalent compounds: Draw the molecular structure of water and label the partial charges of each element. Chemistry worksheet lewis dot structures name:
Ionic And Covalent Bonding Practice Worksheet
Write a simple rule that will allow. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Draw lewis structures for the following covalent compounds: Chemistry worksheet lewis dot structures name:
Covalent Bonding Worksheet
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Draw lewis structures for the following covalent compounds: Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Why is the water molecule’s shape bent and not linear? Chemistry worksheet lewis dot structures name:
Ionic And Covalent Bond Naming Practice
Chemistry worksheet lewis dot structures name: Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Write a simple rule that will allow. Draw the molecular structure of water and label the partial charges of each element. Why is the water molecule’s shape bent and not linear?
Ionic And Covalent Bonding Worksheet With Answers Covalent I
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Draw lewis structures for the following covalent compounds: Write a simple rule that will allow. Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Draw the molecular structure of water and label the partial.
Practice Drawing Covalent Bonds
Draw lewis structures for the following covalent compounds: Why is the water molecule’s shape bent and not linear? Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Chemistry worksheet lewis dot structures name: Write a simple rule that will allow.
Naming Covalent Bonds Worksheet for 9th 12th Grade Lesson
Write a simple rule that will allow. Why is the water molecule’s shape bent and not linear? Draw the molecular structure of water and label the partial charges of each element. Chemistry worksheet lewis dot structures name: Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules.
Covalent Bond Naming Worksheet
Chemistry worksheet lewis dot structures name: Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Write a simple rule that will allow. Draw lewis structures for the following covalent compounds:
Practice Lewis Dot Structure Diagram Lewis Worksheet Dot Str
Write a simple rule that will allow. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Why is the water molecule’s shape bent and not linear? Draw the molecular structure of water and label the partial charges of each element. Draw lewis structures for the following covalent compounds:
Covalent Bond Naming Worksheet
Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Why is the water molecule’s shape bent and not linear? Write a simple rule that will allow. Chemistry worksheet lewis dot structures name: Draw the molecular structure of water and label the partial charges of each element.
Covalent Bonding Practice Worksheet With Answers Printable Online
Draw the molecular structure of water and label the partial charges of each element. Model 1 substances are called ionic compounds and model 2 substances are called covalent molecules. Draw lewis structures for the following covalent compounds: Chemistry worksheet lewis dot structures name: Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8.
Chemistry Worksheet Lewis Dot Structures Name:
Why is the water molecule’s shape bent and not linear? Draw the molecular structure of water and label the partial charges of each element. Draw lewis structures for the following covalent compounds: Write a simple rule that will allow.
Model 1 Substances Are Called Ionic Compounds And Model 2 Substances Are Called Covalent Molecules.
Covalent bonding occurs when two or more non_metals share electrons, attempting to attain a stable octet (8 outer electrons)in their.