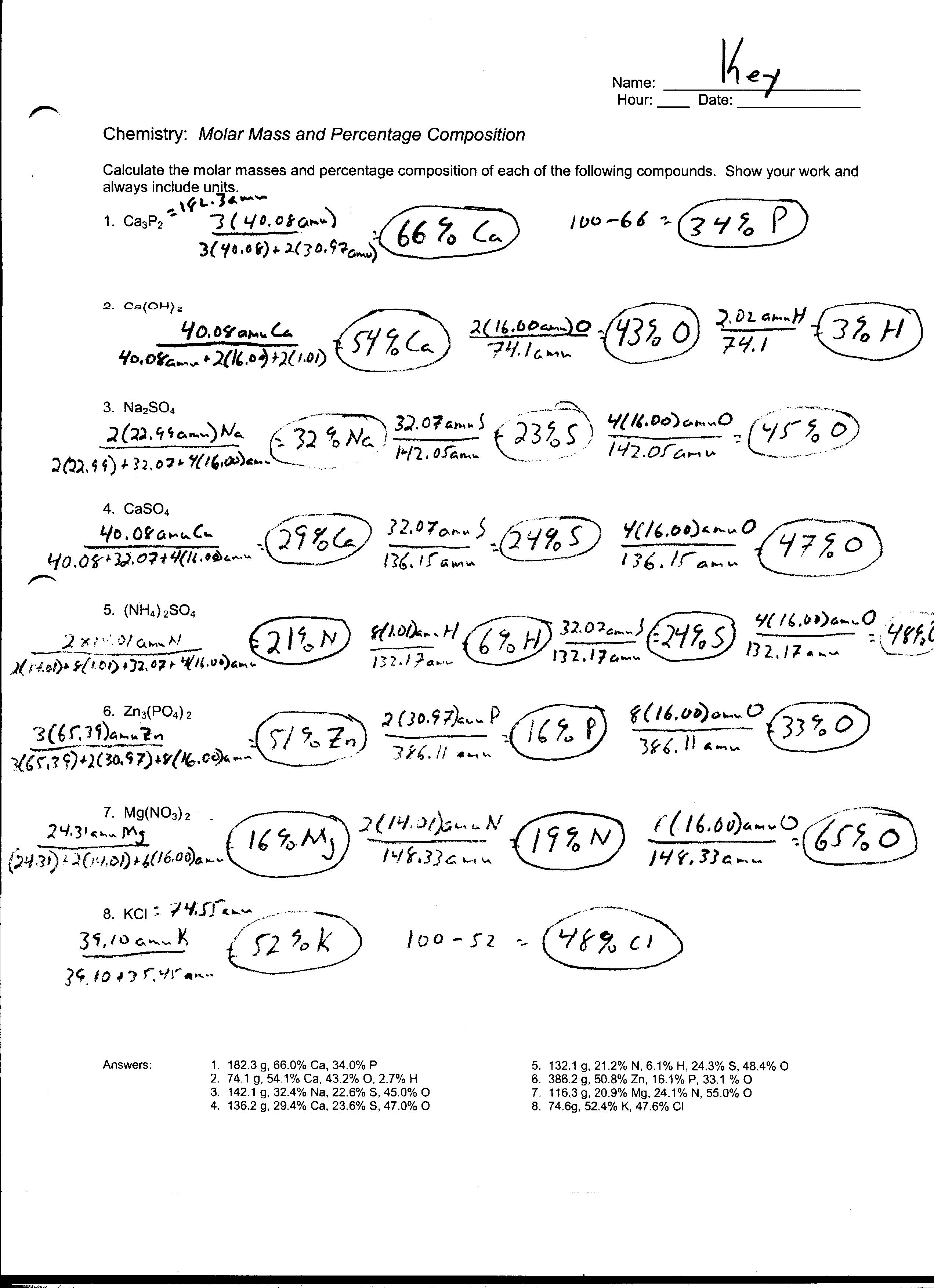
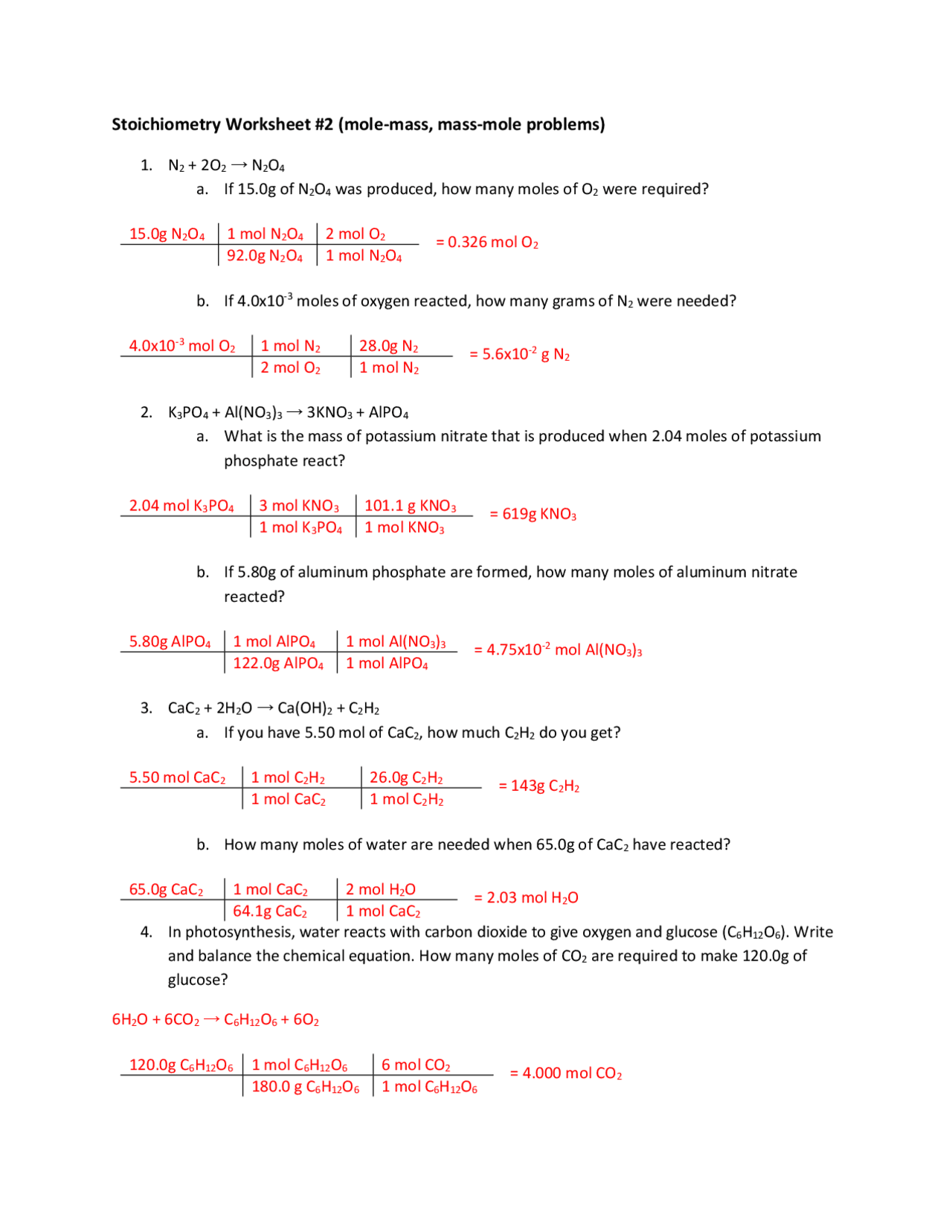
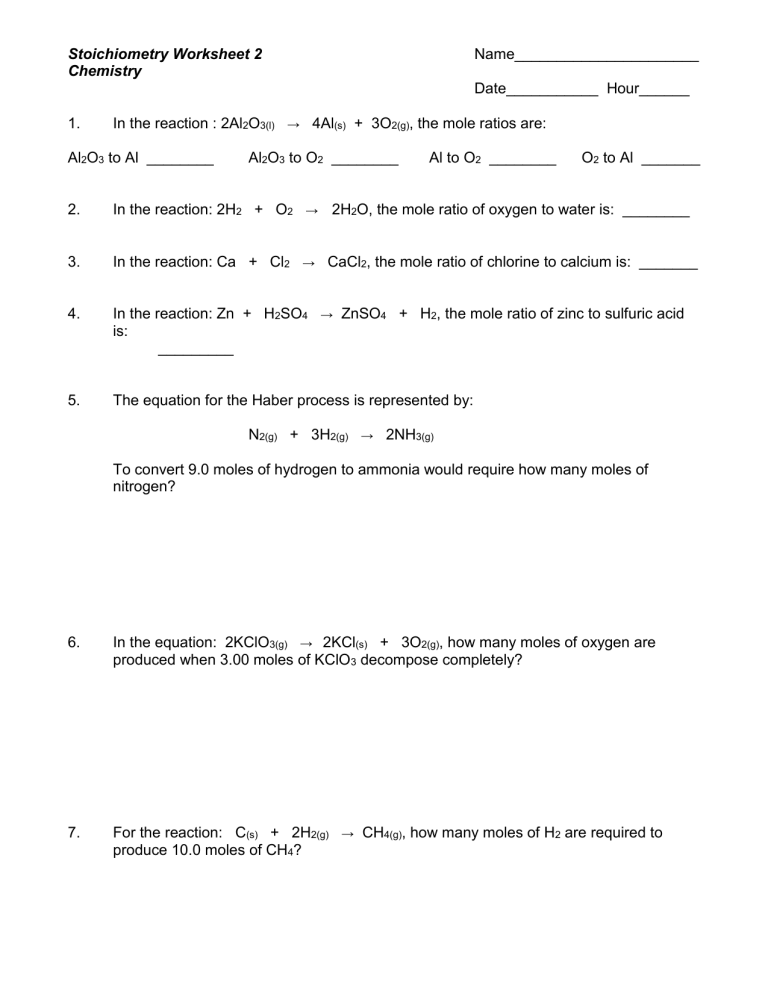
Mole Mole Stoichiometry Worksheet Answers - The atomic weight of carbon is 12.0107 u, so. How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. How many moles of o 2 will be formed from 1.65 moles of kclo 3? How many moles of kclo 3 are needed to make 3.50 moles of kcl? Grams a x 1 mole a x y mole b x g b. Stoichiometry worksheet #1 answers 1.
Stoichiometry worksheet #1 answers 1. How many moles of o 2 will be formed from 1.65 moles of kclo 3? Grams a x 1 mole a x y mole b x g b. How many moles of kclo 3 are needed to make 3.50 moles of kcl? How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. The atomic weight of carbon is 12.0107 u, so.
How many moles of o 2 will be formed from 1.65 moles of kclo 3? Grams a x 1 mole a x y mole b x g b. How many moles of kclo 3 are needed to make 3.50 moles of kcl? How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. The atomic weight of carbon is 12.0107 u, so. Stoichiometry worksheet #1 answers 1.
Mole To Mole Stoichiometry Worksheets With Answers
Grams a x 1 mole a x y mole b x g b. How many moles of kclo 3 are needed to make 3.50 moles of kcl? How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. Stoichiometry worksheet #1 answers 1. How many moles of o 2 will be.
Unit 7 Stoichiometry Mole Conversion Worksheets
How many moles of kclo 3 are needed to make 3.50 moles of kcl? Stoichiometry worksheet #1 answers 1. How many moles of o 2 will be formed from 1.65 moles of kclo 3? The atomic weight of carbon is 12.0107 u, so. Grams a x 1 mole a x y mole b x g b.
Stoichiometry Worksheets 1
How many moles of kclo 3 are needed to make 3.50 moles of kcl? Stoichiometry worksheet #1 answers 1. The atomic weight of carbon is 12.0107 u, so. How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. How many moles of o 2 will be formed from 1.65 moles.
Stoichiometry Part 1 Worksheet Answers
How many moles of kclo 3 are needed to make 3.50 moles of kcl? How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. The atomic weight of carbon is 12.0107 u, so. Grams a x 1 mole a x y mole b x g b. How many moles of.
Solved MoleMole Stoichiometry Worksheet Complete each of
Stoichiometry worksheet #1 answers 1. How many moles of kclo 3 are needed to make 3.50 moles of kcl? How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. The atomic weight of carbon is 12.0107 u, so. Grams a x 1 mole a x y mole b x g.
Mole To Mole Stoichiometry Worksheets
How many moles of o 2 will be formed from 1.65 moles of kclo 3? The atomic weight of carbon is 12.0107 u, so. How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. Grams a x 1 mole a x y mole b x g b. How many moles.
Free Printable Mass to Mole Stoichiometry Worksheets
Stoichiometry worksheet #1 answers 1. How many moles of kclo 3 are needed to make 3.50 moles of kcl? How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. The atomic weight of carbon is 12.0107 u, so. How many moles of o 2 will be formed from 1.65 moles.
Stoichiometry Worksheet With Answer Key printable pdf download
How many moles of o 2 will be formed from 1.65 moles of kclo 3? The atomic weight of carbon is 12.0107 u, so. Grams a x 1 mole a x y mole b x g b. How many moles of kclo 3 are needed to make 3.50 moles of kcl? How many grams of sodium sulfate will be formed.
Mole To Mole Stoichiometry Worksheets
The atomic weight of carbon is 12.0107 u, so. How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. How many moles of o 2 will be formed from 1.65 moles of kclo 3? How many moles of kclo 3 are needed to make 3.50 moles of kcl? Grams a.
Stoichiometry Worksheets And Key Answers
Stoichiometry worksheet #1 answers 1. How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you. How many moles of kclo 3 are needed to make 3.50 moles of kcl? Grams a x 1 mole a x y mole b x g b. The atomic weight of carbon is 12.0107 u,.
How Many Moles Of Kclo 3 Are Needed To Make 3.50 Moles Of Kcl?
Stoichiometry worksheet #1 answers 1. How many moles of o 2 will be formed from 1.65 moles of kclo 3? The atomic weight of carbon is 12.0107 u, so. How many grams of sodium sulfate will be formed if you start with 200 grams of sodium hydroxide and you.