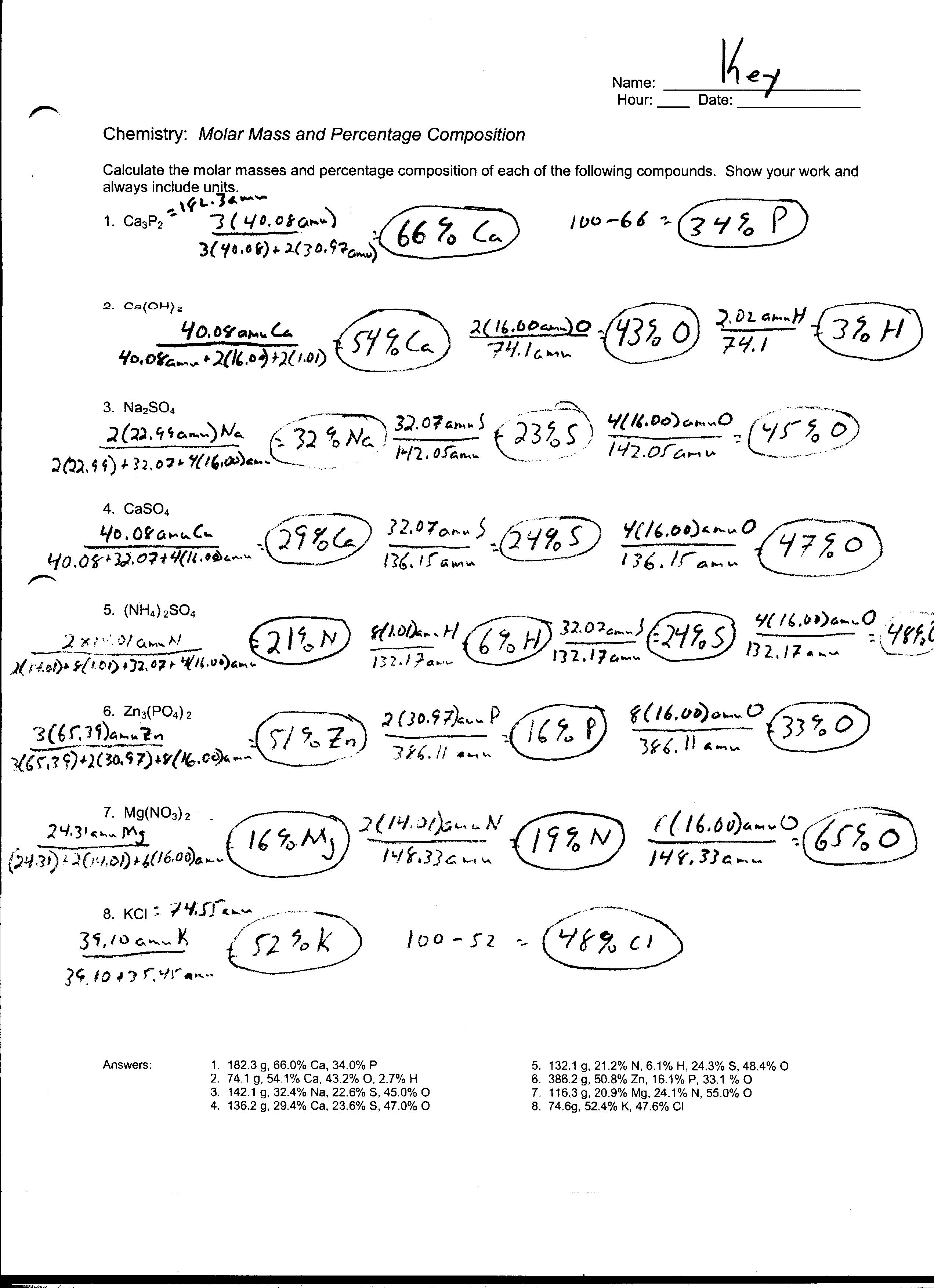
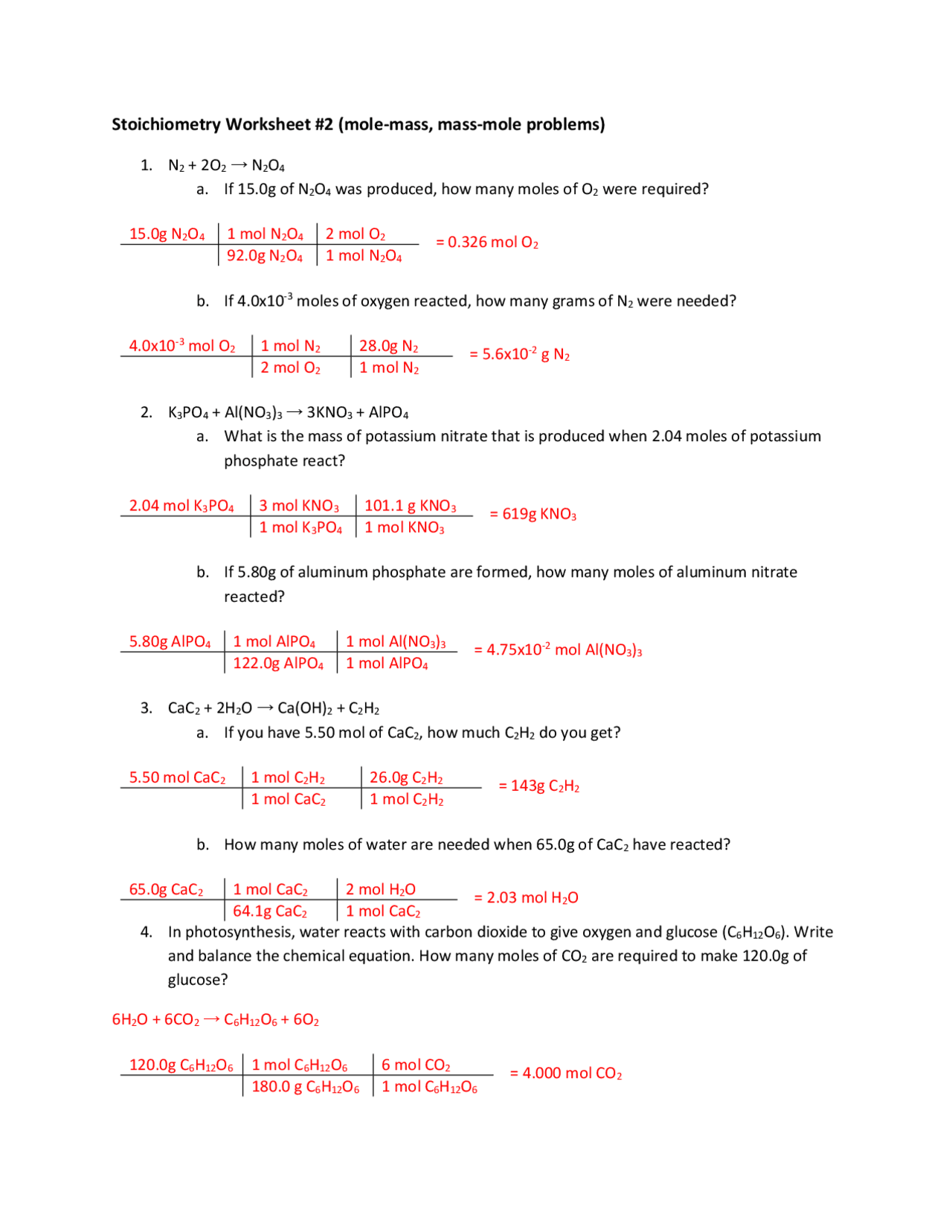
Mole To Mole Stoichiometry Worksheet - Mole to mole problems 1. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Mole to mole problems 1. How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?.
How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?.
Mole Mole Stoichiometry Worksheets
Mole to mole problems 1. How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Mole Mole Stoichiometry Worksheets Answers
Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of magnesium is 3.01 x 1022 atoms of. Mole to mole problems 1.
Mole Stoichiometry Worksheet
Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of magnesium is 3.01 x 1022 atoms of.
Stoichiometry and The Mole Review Worksheet Set (Study Guide
Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. How many moles of magnesium is 3.01 x 1022 atoms of.
Unit 7 Stoichiometry Mole Conversion Worksheets
How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Mole to mole problems 1. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of magnesium is 3.01 x 1022 atoms of.
Mole To Mole Stoichiometry Worksheets
How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Free Printable Mass to Mole Stoichiometry Worksheets Worksheets Library
How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1.
Solved MoleMole Stoichiometry Worksheet Complete each of
Mole to mole problems 1. How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Mole Mole Stoichiometry Worksheets
Mole to mole problems 1. How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Mole To Mole Stoichiometry Worksheets With Answers
How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?. Magnesium reacts with hydrochloric acid according to the following balanced chemical.
Magnesium Reacts With Hydrochloric Acid According To The Following Balanced Chemical.
How many moles of magnesium is 3.01 x 1022 atoms of. Magnesium reacts with hydrochloric acid according to the following balanced chemical. Mole to mole problems 1. How many moles of sodium will react with water to produce 4.0 mol of hydrogen in the following reaction?.