Monitoring Plan Template - This m&e plan template outlines the typical components of an m&e plan. M&e plans are intended to guide monitoring and evaluation. The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial.
This m&e plan template outlines the typical components of an m&e plan. M&e plans are intended to guide monitoring and evaluation. The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial.
The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. This m&e plan template outlines the typical components of an m&e plan. M&e plans are intended to guide monitoring and evaluation. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial.
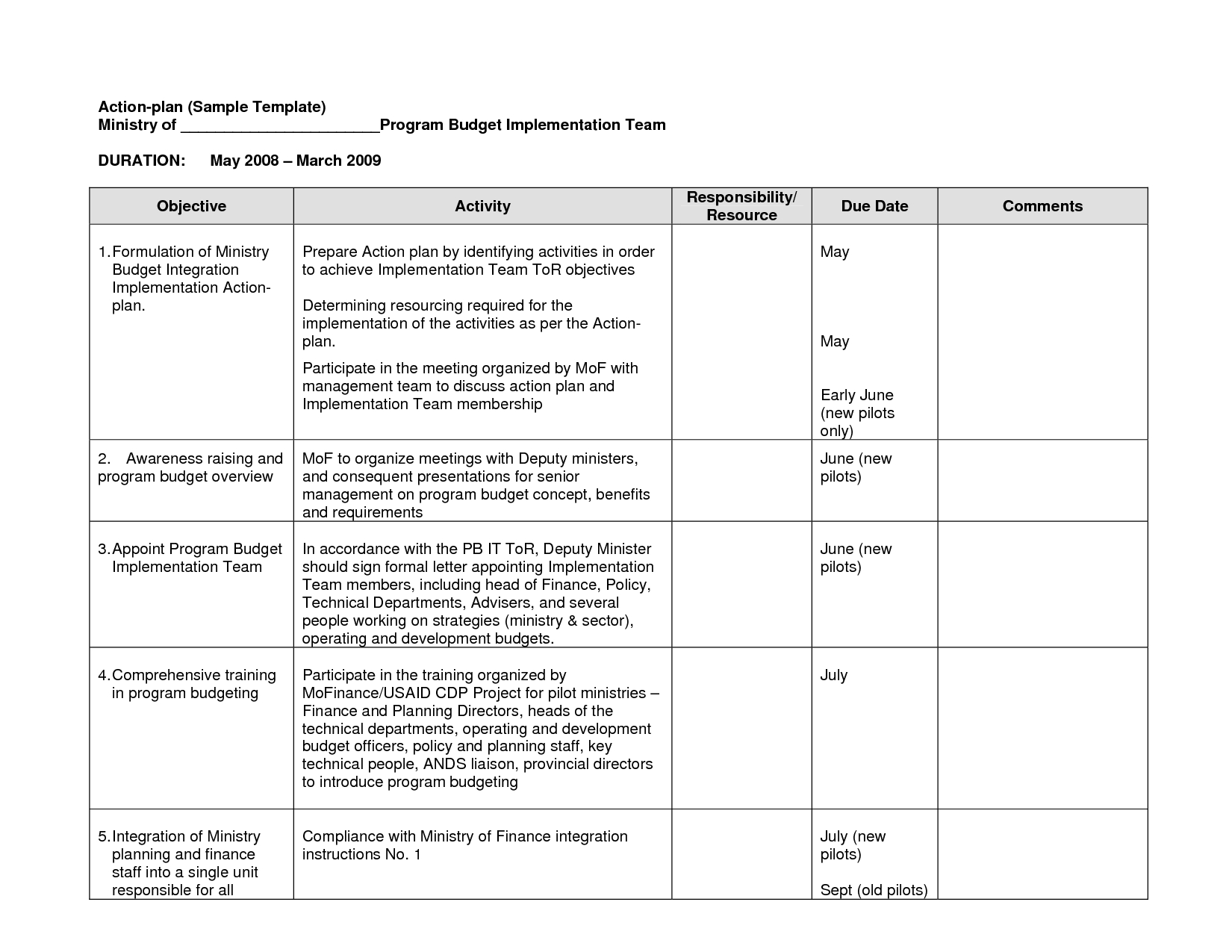
Monitoring Plan Template
This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial. This m&e plan template outlines the typical components of an m&e plan. The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. M&e plans are intended to guide monitoring and.
Rmf Continuous Monitoring Plan Template Master of Documents
The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. M&e plans are intended to guide monitoring and evaluation. This m&e plan template outlines the typical components of an m&e plan. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical.
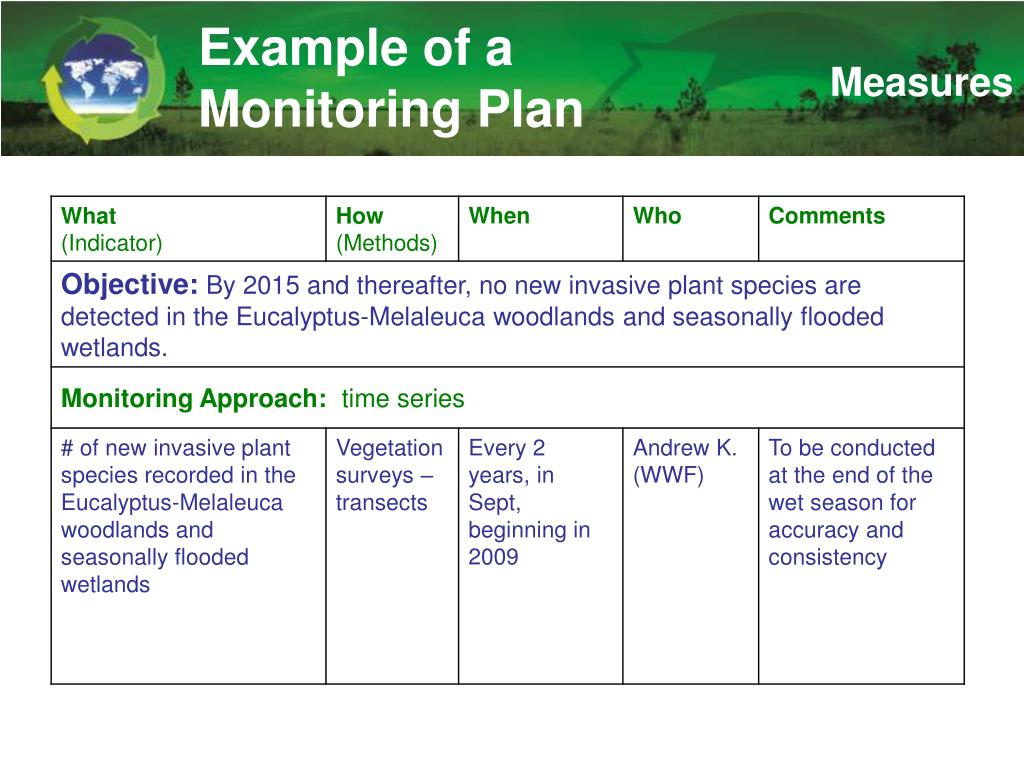
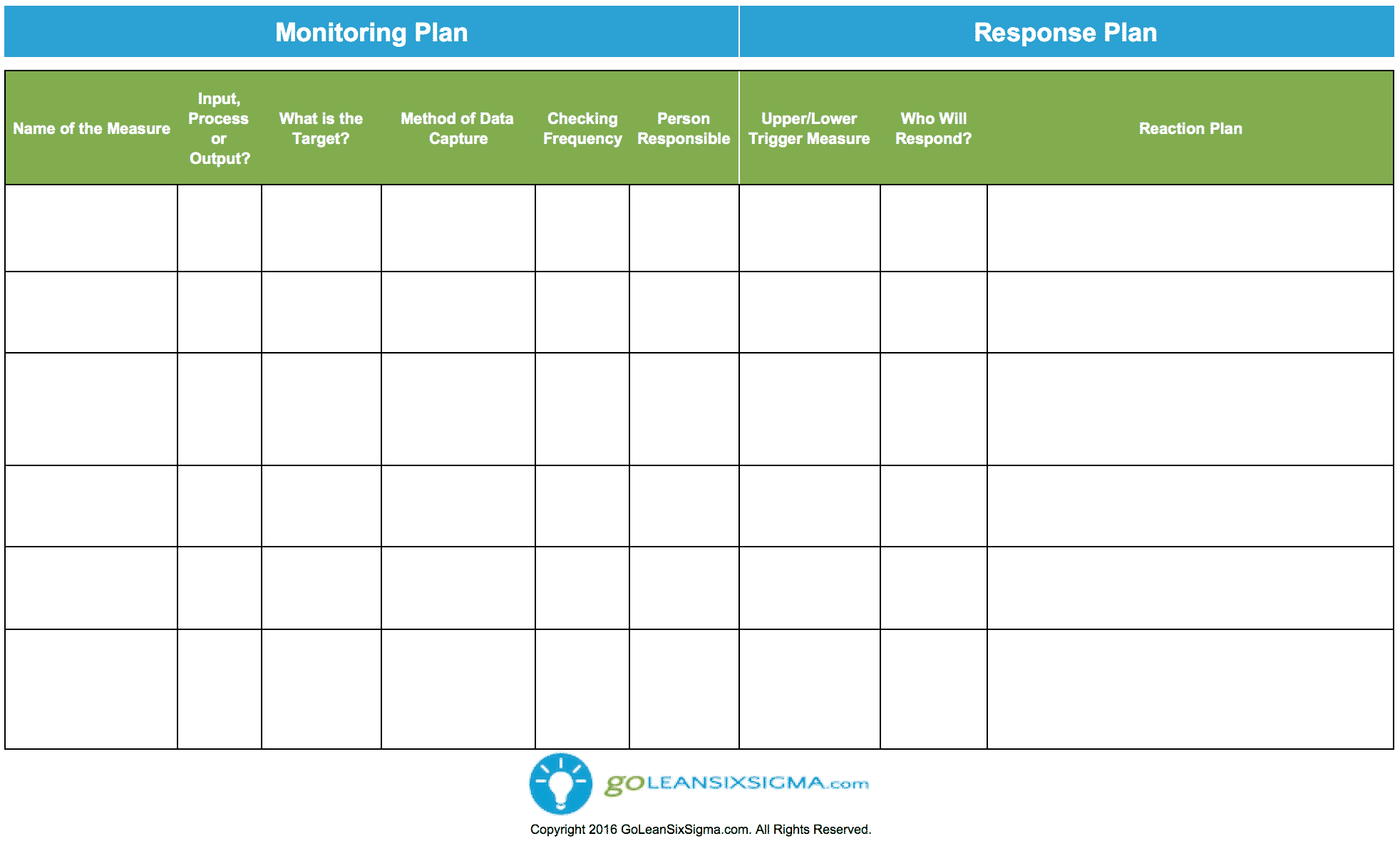
Sample Monitoring Plan
The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial. This m&e plan template outlines the typical components of an m&e plan. M&e plans are intended to guide monitoring and.
Monitoring And Evaluation Report Template Creative Sample Templates
The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. This m&e plan template outlines the typical components of an m&e plan. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial. M&e plans are intended to guide monitoring and.
Monitoring And Evaluation Report Writing Template Professional Template
This m&e plan template outlines the typical components of an m&e plan. The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. M&e plans are intended to guide monitoring and evaluation. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical.
Monitoring Plan Template
The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. M&e plans are intended to guide monitoring and evaluation. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial. This m&e plan template outlines the typical components of an m&e.
Monitoring Plan Template
This m&e plan template outlines the typical components of an m&e plan. The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. M&e plans are intended to guide monitoring and evaluation. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical.
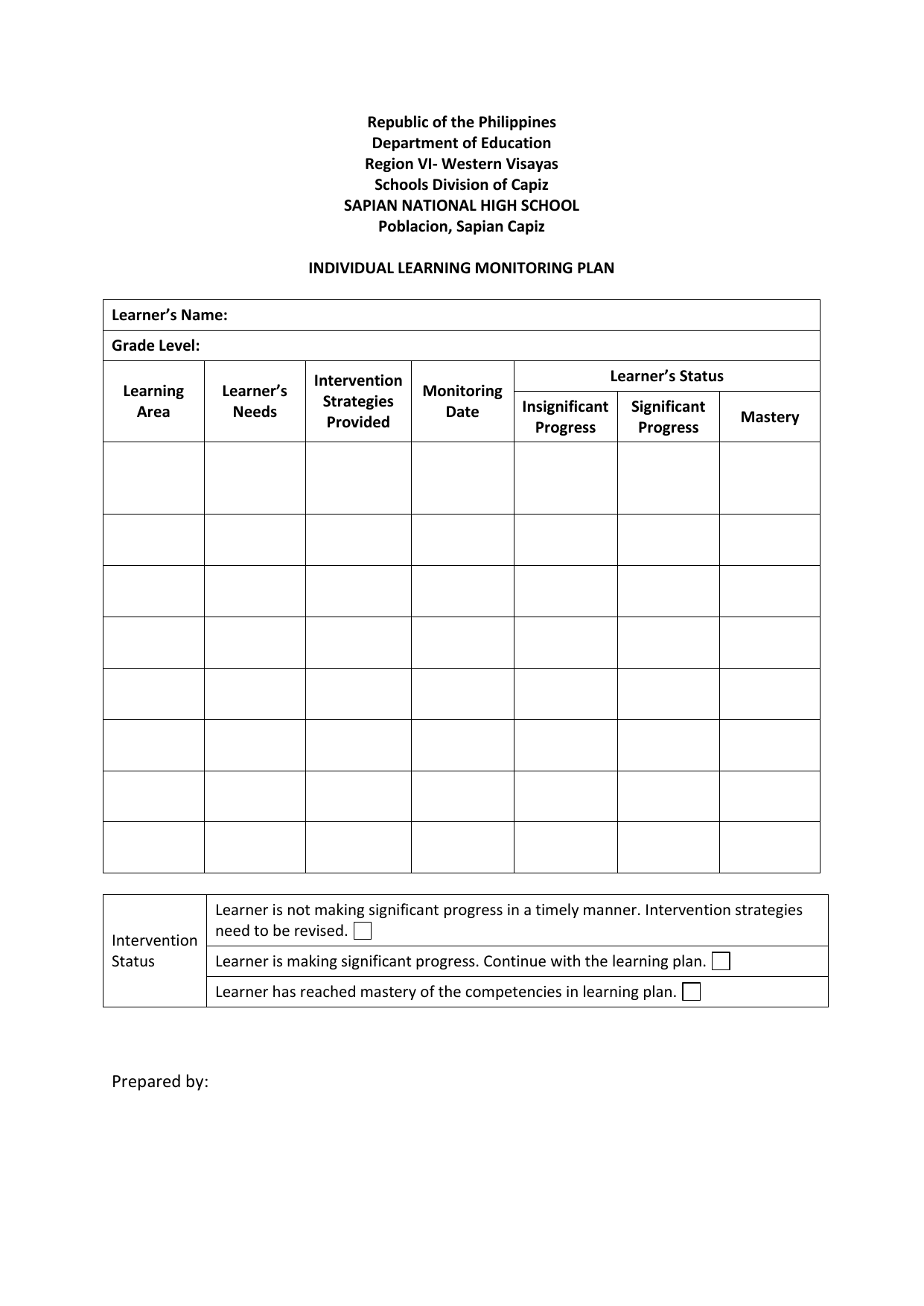
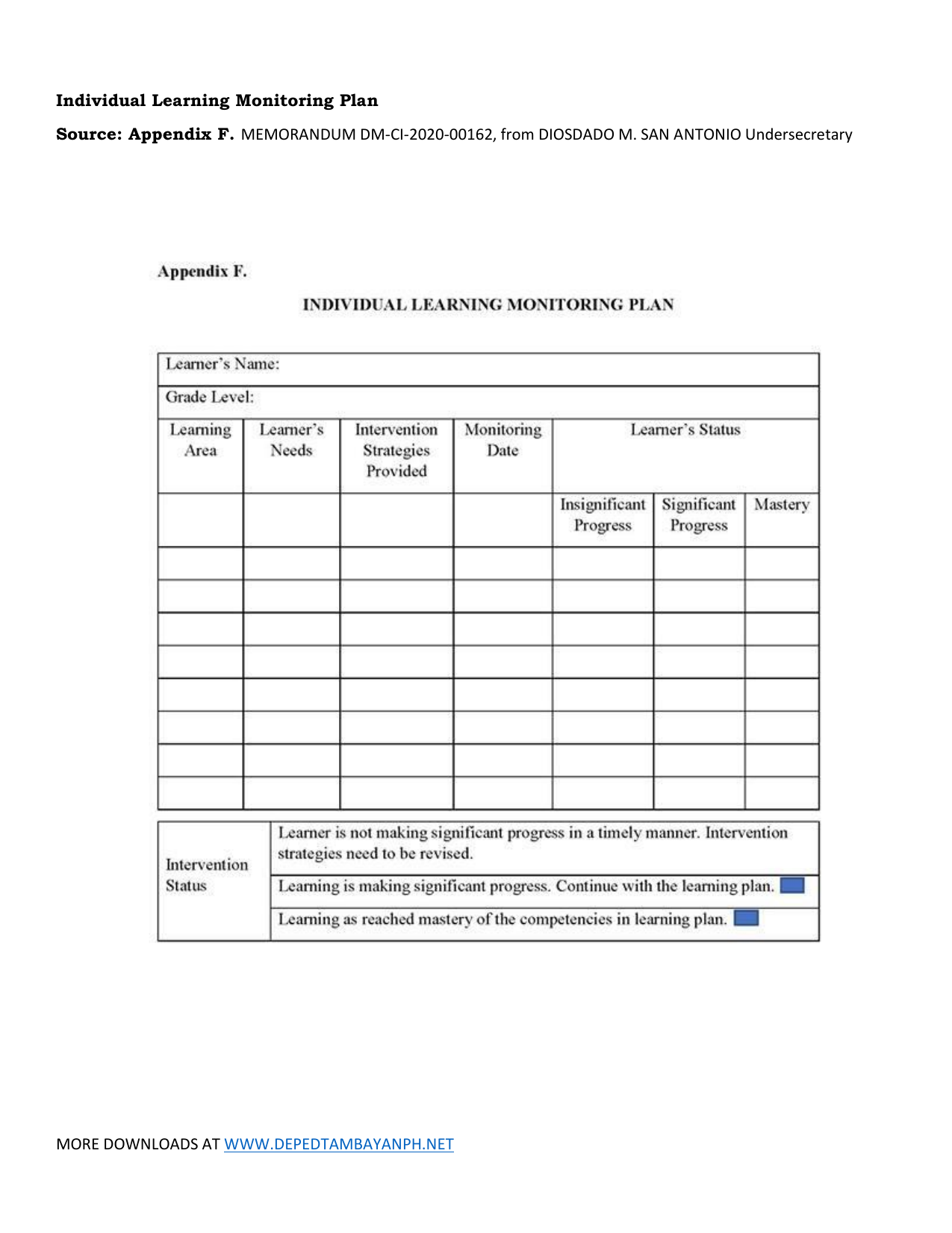
INDIVIDUAL LEARNING MONITORING PLAN SAMPLE & TEMPLATE
This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial. This m&e plan template outlines the typical components of an m&e plan. The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. M&e plans are intended to guide monitoring and.
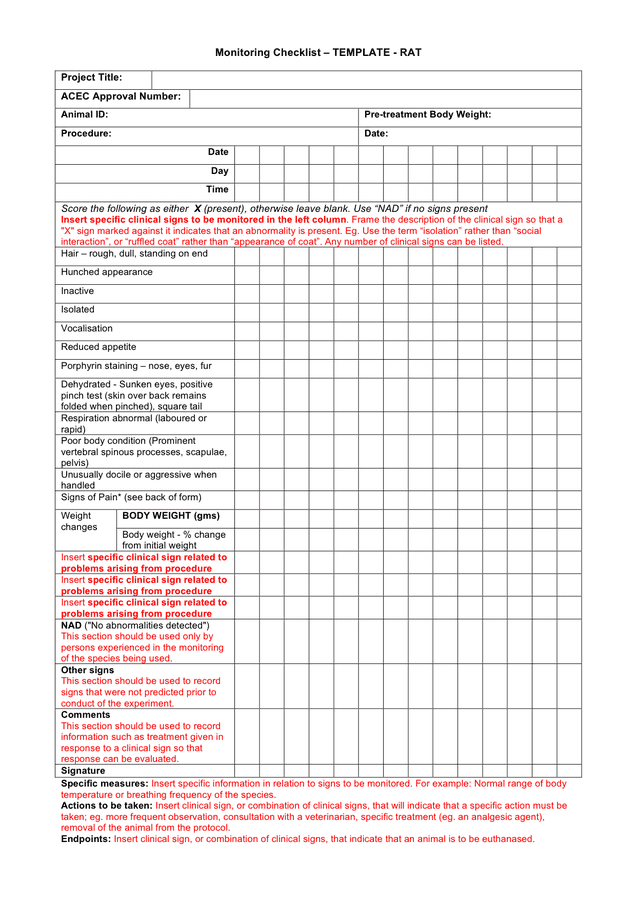
Monitoring checklist template in Word and Pdf formats
This m&e plan template outlines the typical components of an m&e plan. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial. M&e plans are intended to guide monitoring and evaluation. The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to.
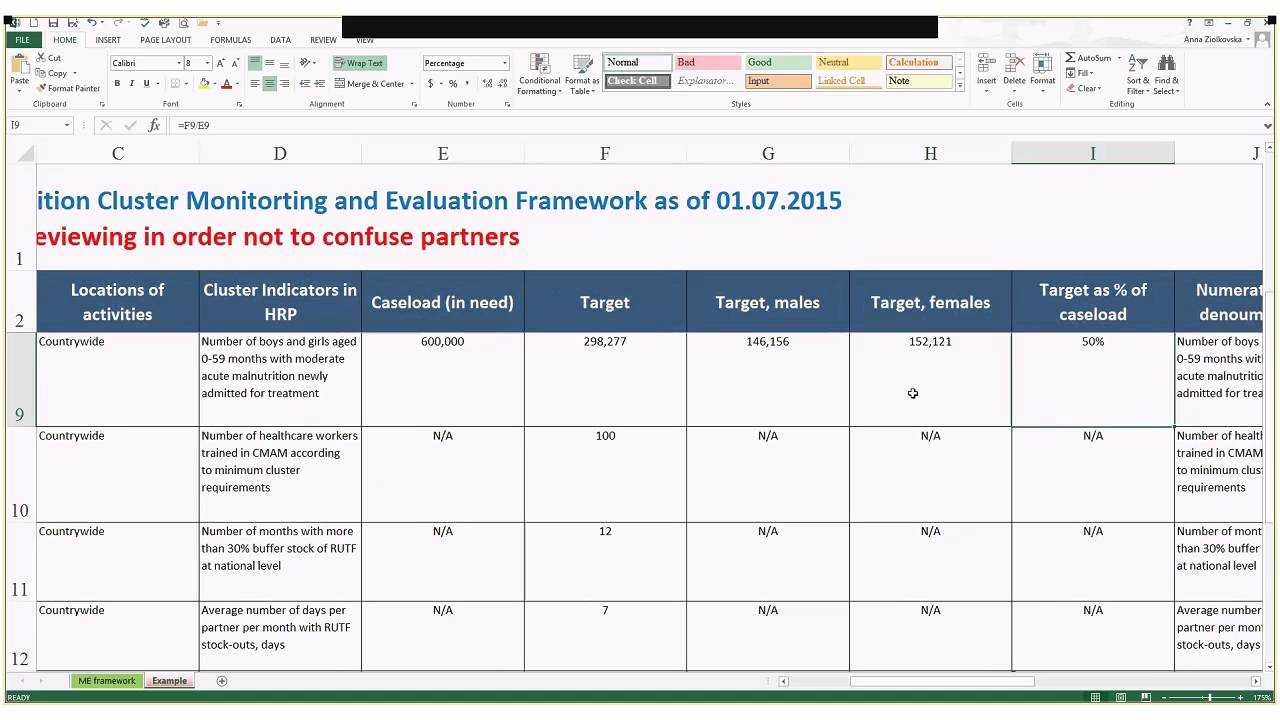
Clinical Trial Monitoring Plan Template
The plan should describe the monitoring strategy, the monitoring responsibilities of each party involved, the various monitoring methods to be. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial. M&e plans are intended to guide monitoring and evaluation. This m&e plan template outlines the typical components of an m&e.
The Plan Should Describe The Monitoring Strategy, The Monitoring Responsibilities Of Each Party Involved, The Various Monitoring Methods To Be.
This m&e plan template outlines the typical components of an m&e plan. M&e plans are intended to guide monitoring and evaluation. This document identifies key monitoring activities and specifies the data to be reviewed over the course of a clinical trial.