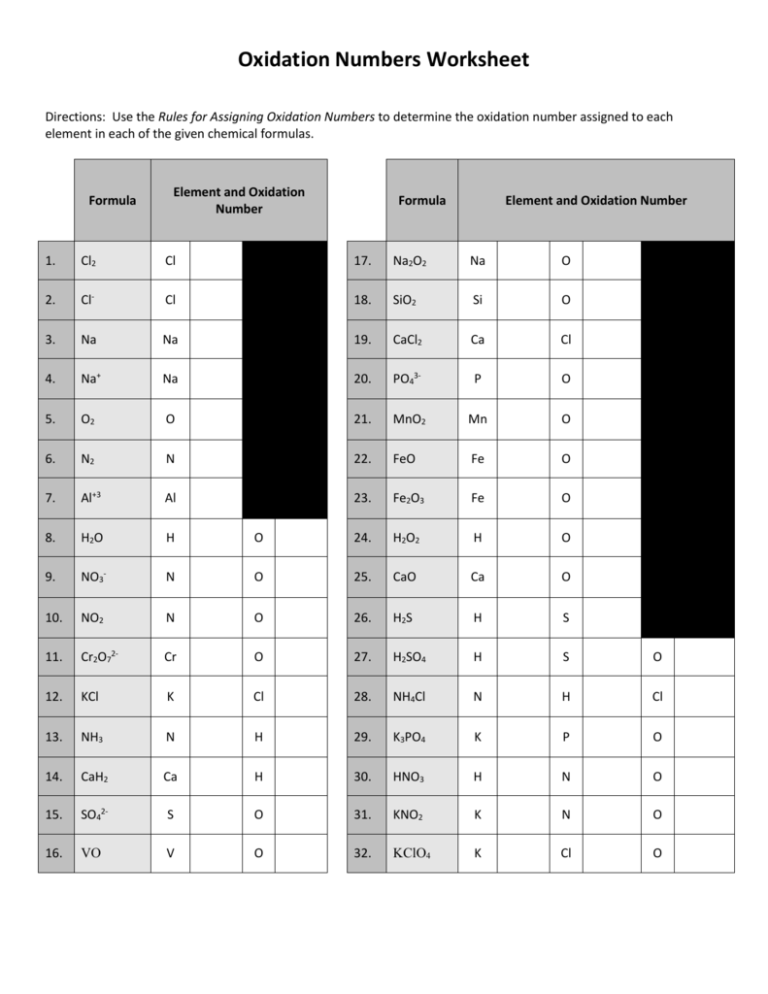
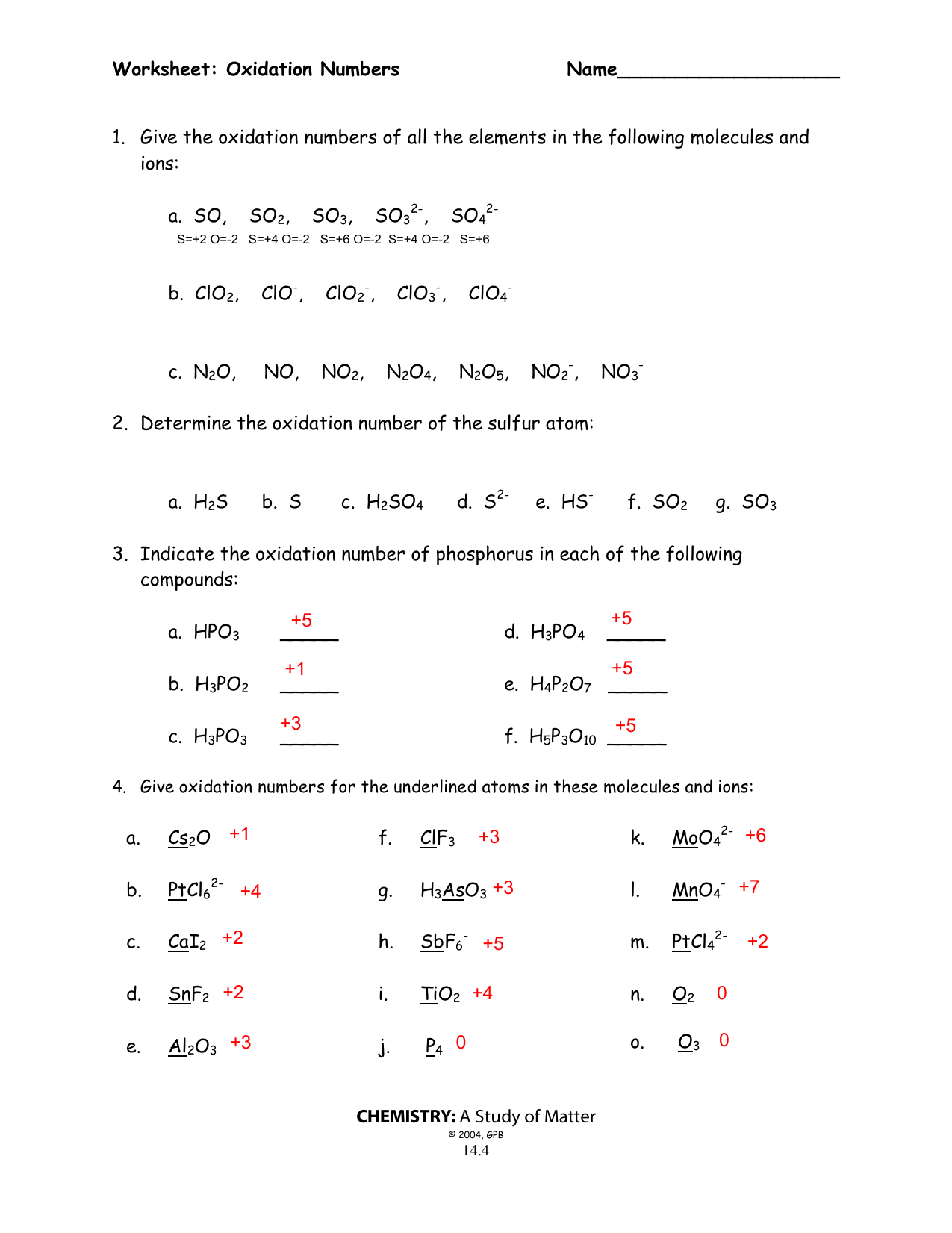
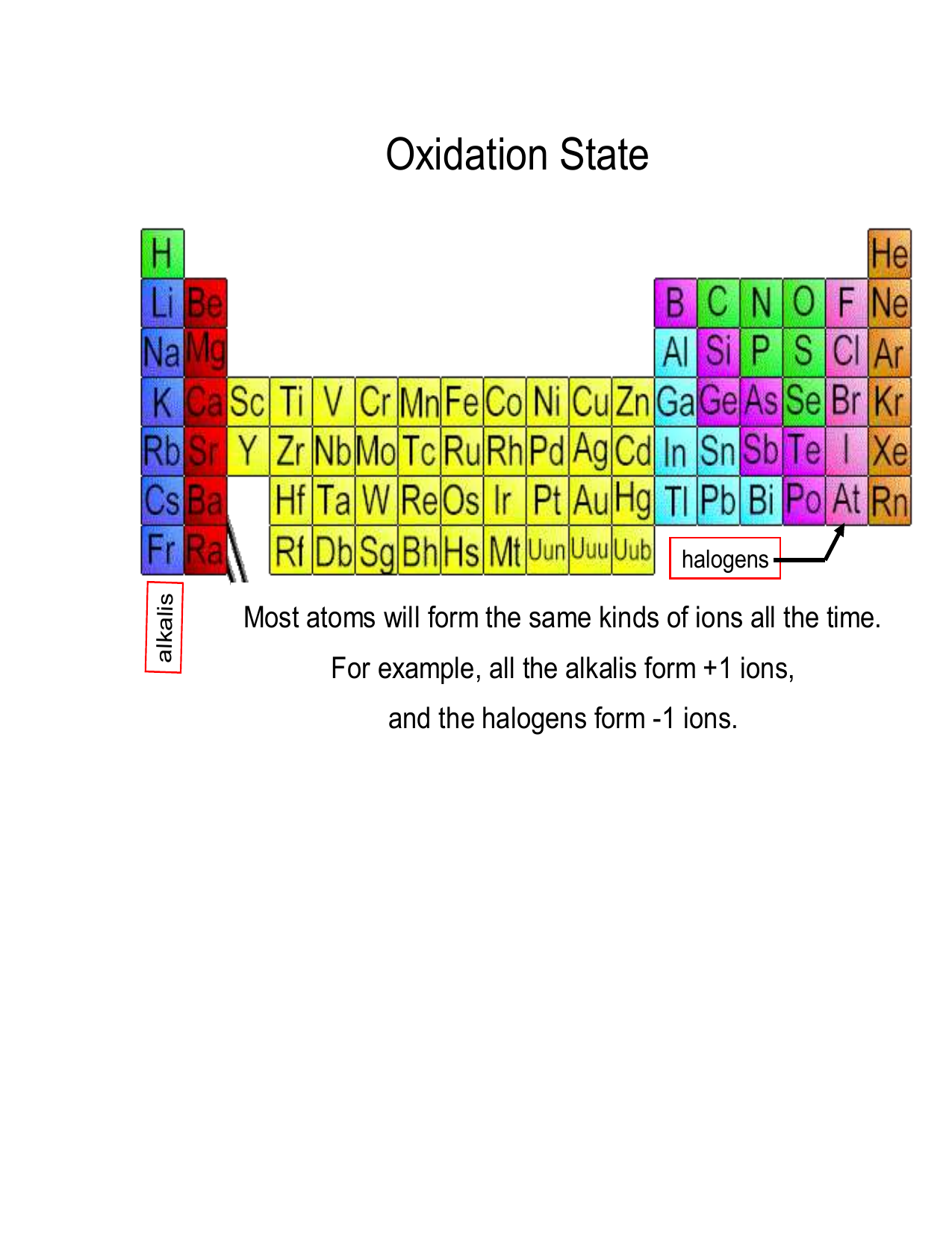
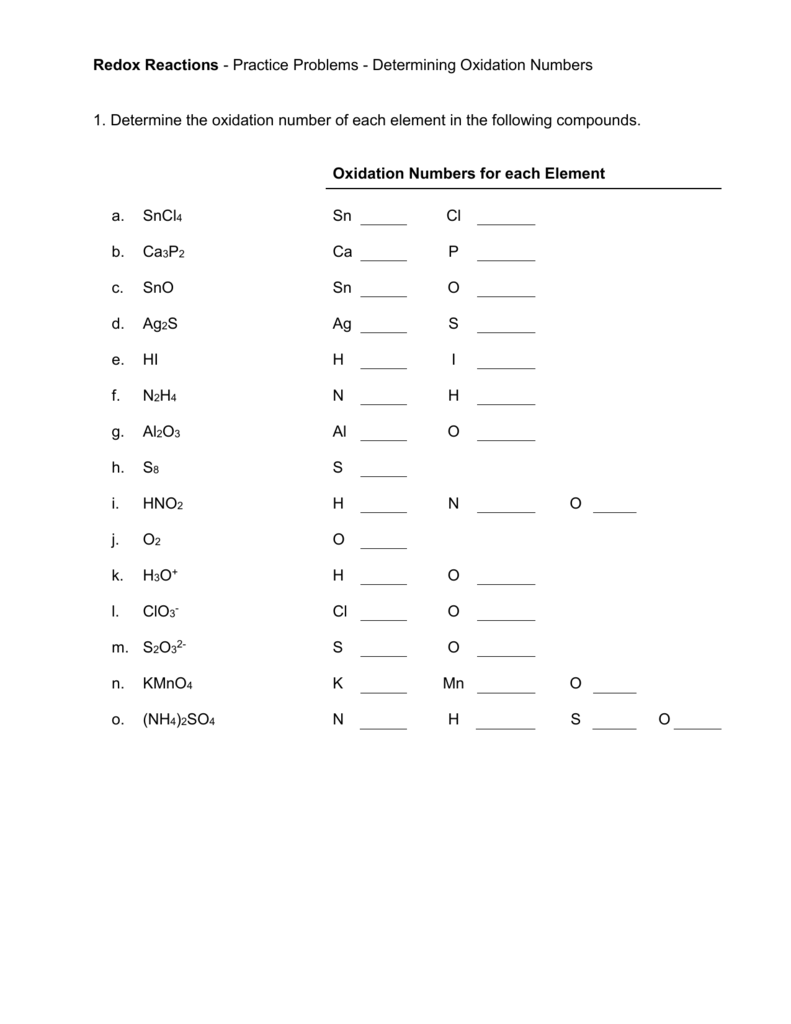
Worksheet Oxidation Numbers - They mark the flow of electrons and are useful for balancing redox (reduction/oxidation). Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. Oxidation numbers are bookkeeping numbers. A pure element has an oxidation number of 0. 2) the oxidation number of a monatomic ion. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. The oxidation number of an element in a monatomic ion equals the. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. In the following questions, give the oxidation number of the indicated atoms/ion.
Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. In the following questions, give the oxidation number of the indicated atoms/ion. The oxidation number of an element in a monatomic ion equals the. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. 2) the oxidation number of a monatomic ion. Oxidation numbers are bookkeeping numbers. They mark the flow of electrons and are useful for balancing redox (reduction/oxidation). A pure element has an oxidation number of 0. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules.
2) the oxidation number of a monatomic ion. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. The oxidation number of an element in a monatomic ion equals the. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. In the following questions, give the oxidation number of the indicated atoms/ion. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. Oxidation numbers are bookkeeping numbers. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. A pure element has an oxidation number of 0. They mark the flow of electrons and are useful for balancing redox (reduction/oxidation).
Oxidation Numbers Worksheet fill in the blank
A pure element has an oxidation number of 0. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. The oxidation number of an element in a monatomic ion equals the. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. They.
Free Printable Oxidation Numbers Worksheets Worksheets Library
A pure element has an oxidation number of 0. The oxidation number of an element in a monatomic ion equals the. In the following questions, give the oxidation number of the indicated atoms/ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing.
Worksheet Oxidation Numbers Answer Key
The oxidation number of an element in a monatomic ion equals the. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. In the following.
Free Printable Oxidation Numbers Worksheets
To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. In the following questions, give the oxidation number of the indicated atoms/ion. Oxidation numbers are bookkeeping numbers. A pure element has an.
With this ColourbyNumber worksheet, chemistry or science students can
The oxidation number of an element in a monatomic ion equals the. In the following questions, give the oxidation number of the indicated atoms/ion. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. 2) the oxidation number of.
Oxidation Numbers Worksheet
Oxidation numbers are bookkeeping numbers. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. The oxidation number of an element in a monatomic ion equals the. 2) the oxidation number of.
Assigning Oxidation Numbers Worksheet 1 Free Worksheets Printable
Oxidation numbers are bookkeeping numbers. A pure element has an oxidation number of 0. 2) the oxidation number of a monatomic ion. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules.
Free Collection of Assigning Oxidation Numbers Worksheets
Oxidation numbers are bookkeeping numbers. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0. The oxidation number of an element in a monatomic ion equals the. To determine the oxidation number of an element in a compound, use all the “known” oxidation states first by applying the above rules. In the following questions, give.
Free Printable Oxidation Numbers Worksheets Worksheets Library
The oxidation number of an element in a monatomic ion equals the. 2) the oxidation number of a monatomic ion. Oxidation numbers are bookkeeping numbers. A pure element has an oxidation number of 0. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0.
07 Finding Oxidation Numbers Worksheet
In the following questions, give the oxidation number of the indicated atoms/ion. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. A pure element has an oxidation number of 0. The oxidation number of an element in a monatomic ion equals the. 2) the oxidation number of.
They Mark The Flow Of Electrons And Are Useful For Balancing Redox (Reduction/Oxidation).
Working out oxidation numbers from electronegativity values, challenging redox questions and comparing the two methods of. In the following questions, give the oxidation number of the indicated atoms/ion. Oxidation numbers worksheet the oxidation number of an atom is the apparent charge assigned to it in a particular molecule, ion or. The oxidation number of an element in a monatomic ion equals the.
To Determine The Oxidation Number Of An Element In A Compound, Use All The “Known” Oxidation States First By Applying The Above Rules.
A pure element has an oxidation number of 0. 2) the oxidation number of a monatomic ion. Oxidation numbers are bookkeeping numbers. Rules for assigning oxidation numbers 1) the oxidation number of any uncombined element is 0.